New Sodium- ion Batteries Could Make Your Smartphone Cheaper and could be the next to Power up Your Phone!
Researchers have recently found an alternative to powering up a phone or any other mobile device for that matter and it has something to so with the salt we use in our food. Yes you read right! Salt! Sodium which is a found in salt can be used in making the next generation battery. This Sodium- ion Batteries are much cheaper as sodium is abundantly found, and much cleaner than lithium-ion batteries that contribute to a lot of pollution.
So the next mobile you buy could be a lot cheaper than you imagine. This sodium- ion battery was discovered by Chongwu Zhou, a professor at USC Viterbi School of Engineering. Salt or sodium which is found in abundance, could mean that scientists could literally walk up to the ocean and get the next best thing or maybe the first best thing to juice up our phones.
Current practice and where does Sodium- ion Batteries Could Make Your Smartphone Cheaper and Cleaner figure in:
Currently, our phones or other devices are being powered up by lithium ion batteries. Lithium causes a lot of pollution and has to be mined from the earth to be got, this makes it a very expensive component in a battery.
Sodium on the other hand is much cleaner than lithium and can be got easily as compared to lithium which makes it a much more viable option than current practice.
As for charging speeds, sodium- ion can charge up to 50% in just two minutes. But scientists are not satisfied yet, they are planning on boosting up charging efficiency and the life of these sodium ion batteries, before bring out sodium- ion batteries to the world.
Problems of using sodium- ion batteries:
In our current batteries lithium ions are stored in graphite- a soft mineral of carbon, but this causes problems for sodium- ions. Sodium – ions cannot be stored in graphite as they are too big.
The Solution to using Sodium- ion Batteries:
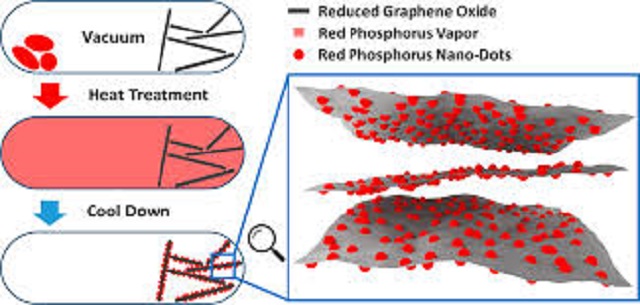
Instead of storing sodium- ions in graphite, researchers thought of another method that would be equally feasible. They decided to arrange red phosphorous on sheets of graphene- which is a layer of honeycombed carbon atoms which is the strongest material ever discovered.
The result, Wavy sheets of something known as “nanodots”. These nanodots can store and also release sodium- ions thus helping scientists bring sodium- ion batteries closer to reality.
Red Phosphorus as an anode in sodium- ion batteries:
Using red phosphorus as an anode in sodium- ion batteries means that it has a high capacity which is theoretically 2596 mAh/g. But using red phosphorus as an anode in a sodium- ion battery means that sodiation and desodiation in a sodium-ion battery causes large volume expansion and also another factor is that it has low electronic conductance.
So to solve the challenges of using red phosphorous as an anode, researchers have used red phosphorus with reduced graphene oxide sheets to make an anode for sodium- ion batteries.